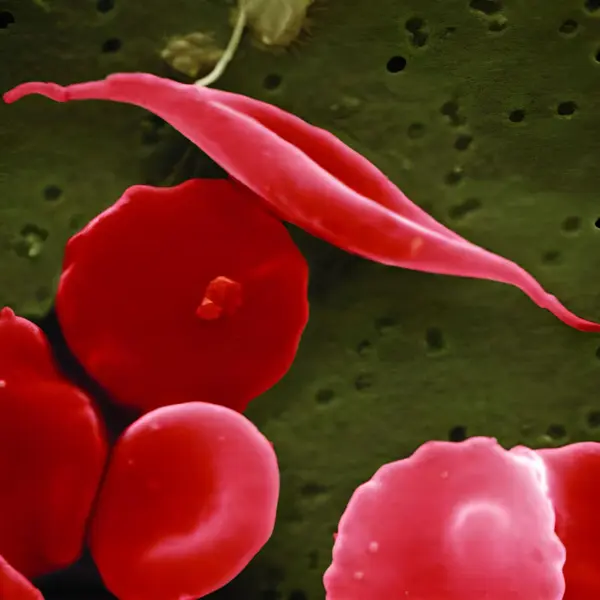
The United States Food and Drug Administration (FDA) on Friday announced the approval of Casgevy, the first CRISPR gene-editing therapy for sickle cell disease, a debilitating genetic illness that affects over 100,000 Americans, the majority of whom are Black.
It is the first gene editing therapy ever to be used in humans, for sickle cell disease. The agency also approved a second treatment using conventional gene therapy for sickle cell that does not use gene editing.
Join our WhatsApp ChannelThis development paves the way for thousands of patients in the United States to receive a treatment that has been described as a “functional cure” for eligible patients. The approval marks the first of two potential breakthroughs for the inherited blood disorder.
The gene editing treatment, called Exa-cel and using the brand name Casgevy, was jointly developed by Vertex Pharmaceuticals of Boston and CRISPR Therapeutics of Switzerland. It uses CRISPR, the Nobel Prize-winning gene editing tool, to snip patients’ DNA. For a small number of subjects in clinical trials, it corrected the effects of the mutation, which results in red blood cells that are shaped like sickles or crescents that become caught in blood vessels, blocking them.
The other approved therapy, called Lyfgenia and made by Bluebird Bio of Somerville, Mass., uses a common gene therapy method to add a good haemoglobin gene to patients’ DNA. Bluebird has 27 authorized centres and also plans to add more.
Both treatments work by genetically modifying a patient’s own stem cells. Until now, the only known cure for sickle cell disease was a bone marrow transplant from a donor, which carries the risk of rejection by the immune system, in addition to the difficult process of finding a matching donor.
Casgevy, which was approved for people ages 12 and older, removes the need for a donor. Using CRISPR, it edits the DNA found in a patient’s stem cells to remove the gene responsible for the devastating blood disorder caused by a single mutated gene.
“It’s a game-changer,” said Dr. Asmaa Ferdjallah, a pediatric hematologist and bone marrow transplant physician at the Mayo Clinic in Rochester, Minnesota.
“To really reimagine and re-discuss sickle cell disease as a curable disease and not as this painful and debilitating chronic disease is hope enough with this news.”
“This could be an equalizer for people with sickle cell because many patients cannot pursue career options” because of the illness.
“It’s something families have been aware of in the early research stage, and they’ve been very patiently waiting for years,” NBC News reports Ferdjallah as saying.