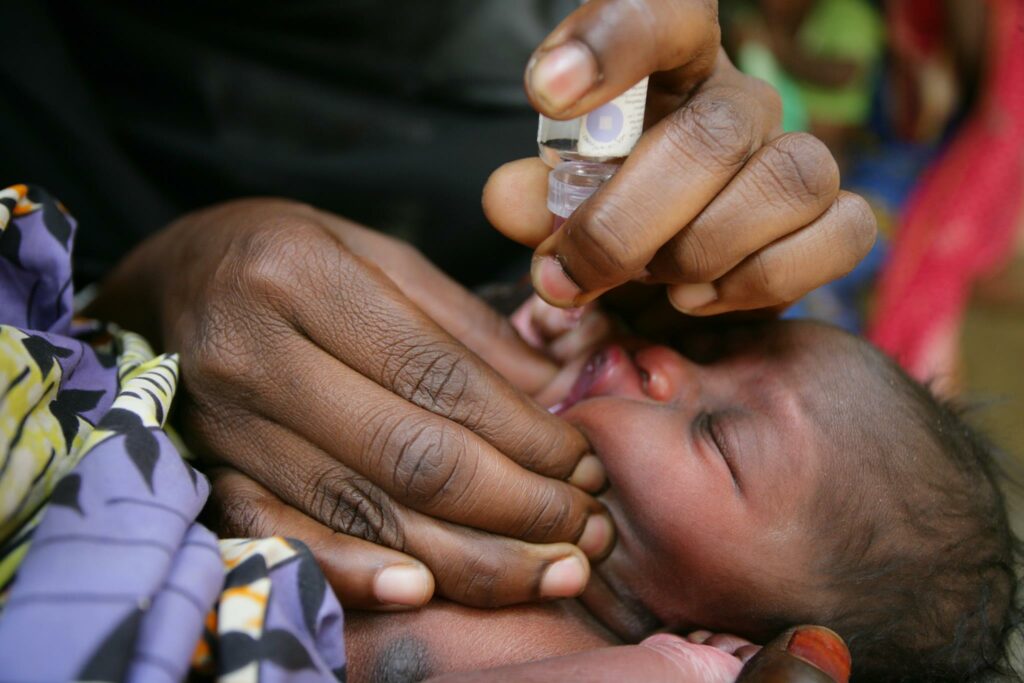
The World Health Organization (WHO) has expressed deep concern over the recent deaths of at least 20 children in India linked to contaminated cough syrups, highlighting significant gaps in the country’s drug safety regulations. The contaminated syrups, containing diethylene glycol (DEG), a toxic substance found in industrial solvents, have raised serious questions about India’s pharmaceutical quality control.
Contaminated Cough Syrups: The Culprits Behind the Tragedy
Three cough syrups have been identified as contaminated with DEG: They are Coldrif (Sresan Pharmaceuticals), linked to the majority of the deaths, particularly in Madhya Pradesh, where children under five succumbed to kidney failure after consuming the syrup; Respifresh (Rednex Pharmaceuticals), found to contain DEG, although not directly linked to any fatalities; and ReLife (Shape Pharma), also contaminated with DEG, with no reported deaths.
Join our WhatsApp ChannelIndia’s Response: Action Against the Manufacturers
The Indian government has already taken swift action, arresting the owner of Sresan Pharmaceuticals, G Ranganathan, and ordering a halt to production. The Tamil Nadu Health Minister has stated that the company’s manufacturing license will be permanently canceled. Additionally, many states have banned these cough syrups, while some have prohibited the use of all cough and cold syrups for children under two years old.
READ ALSO:Rising Suicide Rates In Ghana Prompt National Call For Mental Health Action
World Mental Health Day 2025: Focus Shifts to Mental Health in Humanitarian Crises
Climate Change Chaos: Africa Emits Less But Suffers More
Regulatory Gaps: A Global Concern
Prime Business Africa reports that the WHO has warned that contaminated medicines could reach other countries through unregulated distribution channels, highlighting the need for improved international cooperation and oversight. The global health body has urged India to ensure that such incidents do not recur, emphasizing the importance of robust quality control measures.
Investigation and Inspection Findings
A recent inspection by the Tamil Nadu Drug Control department revealed that Sresan Pharmaceuticals violated 364 manufacturing rules, including poorly qualified staff, substandard water and equipment, lack of pest control, missing production monitoring procedures and no quality assurance or data collection department
It was also found that the company stored products in unhygienic conditions, discharged sewage without purification, and stored water for drug production in an unhygienic manner.
A Pattern of Concern: India’s Pharmaceutical Industry
This incident is not isolated, as Indian-made cough syrups have faced global scrutiny in recent years. In 2023, contaminated syrups were linked to the deaths of 70 children in The Gambia and 18 in Uzbekistan. The incident has raised concerns about India’s quality control mechanisms and the need for stricter regulations.
The WHO’s concerns and the government’s actions signal a critical moment for India’s pharmaceutical industry, which has faced scrutiny over quality control in the past. Ensuring the safety and efficacy of medicines is paramount, particularly for vulnerable populations like children.
- Editor
- Editor
- Editor
- Editor
- Editor